Graduate students (Masters or PhD) who wish to obtain the CLSA data for the sole purpose of their thesis, and postdoctoral or clinical fellows (limit 1 waiver per fellowship) who wish to obtain the CLSA data for the sole purpose of their postdoctoral or clinical fellowship project, who are enrolled at Canadian institutions for their graduate degree, postdoc, or clinical fellowship, can apply for a fee waiver. This fee waiver only applies to alphanumeric data. Any additional data requested, such as images and raw data, have associated fees. The trainee fee waiver cannot be applied to CIHR catalyst grant applications. Canadian trainees working outside Canada but funded through a Canadian source are also eligible for a fee waiver. The request for a fee waiver must be checked in Part 1 of the CLSA Data and Biospecimen Access Request Application in Magnolia.
Apply for Data Access
Requests for data access are processed via the CLSA’s online application system, Magnolia, excluding those involving administrative health data, which are facilitated by HDRN Canada. More information on that application process is available here.
The CLSA data are currently available to approved public sector researchers in Canada and elsewhere, with no preferential or exclusive access for any individual.
For trainee applications, the primary applicant must be the supervisor, on behalf of the trainee. The supervisor is responsible for the content and final submission of the application and closely works with the trainee to prepare the application. As primary applicant, the supervisor must also request a Magnolia user account on behalf of the trainee.

How it Works
Learn who is eligible and how to apply for CLSA data access.
Interested in accessing administrative health data?
Researchers can apply to access linked CLSA cohort data at select provincial data centres. Applications for multiregional data must be submitted through Health Data Research Network (HDRN) Canada’s Data Access Support Hub (DASH).
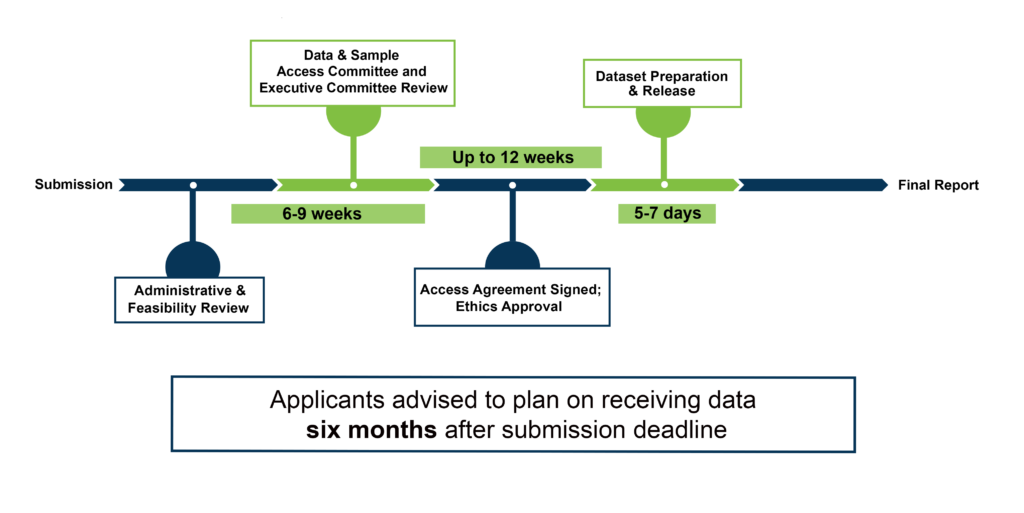
Data Access Timeline
Fees
The CLSA charges researchers using a partial cost-recovery model. The fees incorporate partial recovery of administrative costs, data processing, data retrieval, and delivery costs incurred by the CLSA as part of the data and biospecimen access process.
The charge for an approved application is $3,000 CAD for researchers based in Canada, and $5,000 USD for researchers based at institutions outside of Canada. Additional fees apply for access to image files, raw data and datasets that require more complex customization.
The cost for any other raw data will be determined on a case-by-case basis. Fees are payable per wave when requesting these data in addition to the regular data access application fee.
A fee waiver (some restrictions apply) will be granted to trainees if the CLSA dataset requested will be for the sole use of the graduate student’s thesis research or the postdoctoral or clinical fellow’s research project (limit one waiver per fellow). For more information on eligibility, visit Trainees.
| Data Access Application Fee | Within Canada $ 3,000 CAD | Outside Canada $5,000 USD |
|---|---|---|
| Additional data | Type of data | Additional fee* |
| cIMT | Still image (dicom) | $500 |
| Cineloops (dicom) | $3,000** | |
| DXA | Forearm (image-jpg) | $500 |
| Hip (image-jpg) | $500 | |
| Whole body (image-jpg) | $500 | |
| IVA Lateral Spine (image-dicom) | $500 | |
| ECG | Raw+ (ECG Waveforms) | $500 |
| Images (ECG Tracing-jpg) | $500 | |
| Retinal Scan | Retinal Scan (image-jpg) | $500 |
| Spirometry | RAW+ (Flow + Volume curves – text) | $500 |
| Images (Summary Report-pdf)*** | $500 | |
| Tonometry | Pressure and applanation data | N/A |
| Cognition | Raw data (individual scores) | N/A |
| * Fee payable ** Fee payable for Baseline and/or Follow-up 1 when requested at the same time. *** Not available for Baseline. |
||
| as of 2023Oct30 | ||
Application Deadlines
All applications must be received by 5 p.m. Eastern Time on the day of the submission deadline.
| Submission Deadline | Anticipated Notification of Decision* |
|---|---|
| October 2, 2024 | January 2025 |
| January 15, 2025 | April 2025 |
| April 9, 2025 | July 2025 |
| July 9, 2025 | October 2025 |
| October 1, 2025 | January 2026 |
| *Please note that Anticipated Notification of Decision refers to the approximate time you will be notified about the approval status of your project, and not to the time you will have access to the data. | |
Frequently Asked Questions
Answers to our most frequently asked questions about data access, datasets, applying to the CLSA research platform, using the DataPreview Portal, and presentations and publications.
Once you submit your CLSA Data and Biospecimen Request Application, you can track the progress of your application through Magnolia, the online application system. Once you have begun the application process, it is your responsibility to check your email (including your folders for junk, spam, etc.) for notifications from Magnolia. You may be contacted by the Data Access Team, if additional information is required. You will be notified about the approval status of your application approximately three months after the submission deadline. If your application is approved, a CLSA Access Agreement must be negotiated and signed between McMaster University and your institution. This part of the process can take a variable length of time (up to an additional three (3) months) and is not under the control of the CLSA. You will also need to provide evidence of ethics approval for your project, if you had not done so within your initial application. Please be aware that these steps may affect the length of time that it takes for the data to be released to you. Once all parties have signed the CLSA Access Agreement and proof of ethics approval has been received by the CLSA, your data will be released within 10 working days. Please note that the release of additional data (images and raw data) will require more time (one to three months, depending on the request). When planning for your project, you must include in your timeframe at least six (6) months from the application submission deadline to the time you receive your dataset.
Yes. Please note that ethics approval is not required at the time of the application to use CLSA data, but no data or biospecimens will be released until proof of ethics approval has been received by the CLSA. Should your institution not require a full ethical review for the use of de-identified data, please provide a letter from your Institutional Review Board to this effect. Ethics approval must be obtained only from the primary applicant’s institution, not from all of the institutions of the members of the project team.
The CLSA DataPreview Portal has been designed to help researchers browse available variables in the CLSA dataset and find basic frequencies. Should you need additional information not available through the DataPreview Portal to determine the feasibility of your proposal, please contact us at access@clsa-elcv.ca. We can provide simple cross-tabulations (of two or three variables) from cross-sectional data. While we do our utmost to respond to data queries to help potential users ensure that their proposal is feasible, please note that we are not resourced to provide statistics on change across time-points or more in-depth statistical support.
Should you encounter any issues completing the online application, please contact us via access@clsa-elcv.ca.
Data Availability
Baseline, Follow-up 1, Follow-up 2 data are available to approved public sector researchers in Canada and elsewhere. Researchers can also apply to access data from the COVID-19 studies.
Data Preview Portal
The DataPreview Portal (DPP) is a variable search tool designed to enable researchers to locate items of interest within available data. The CLSA DPP is available in English only.